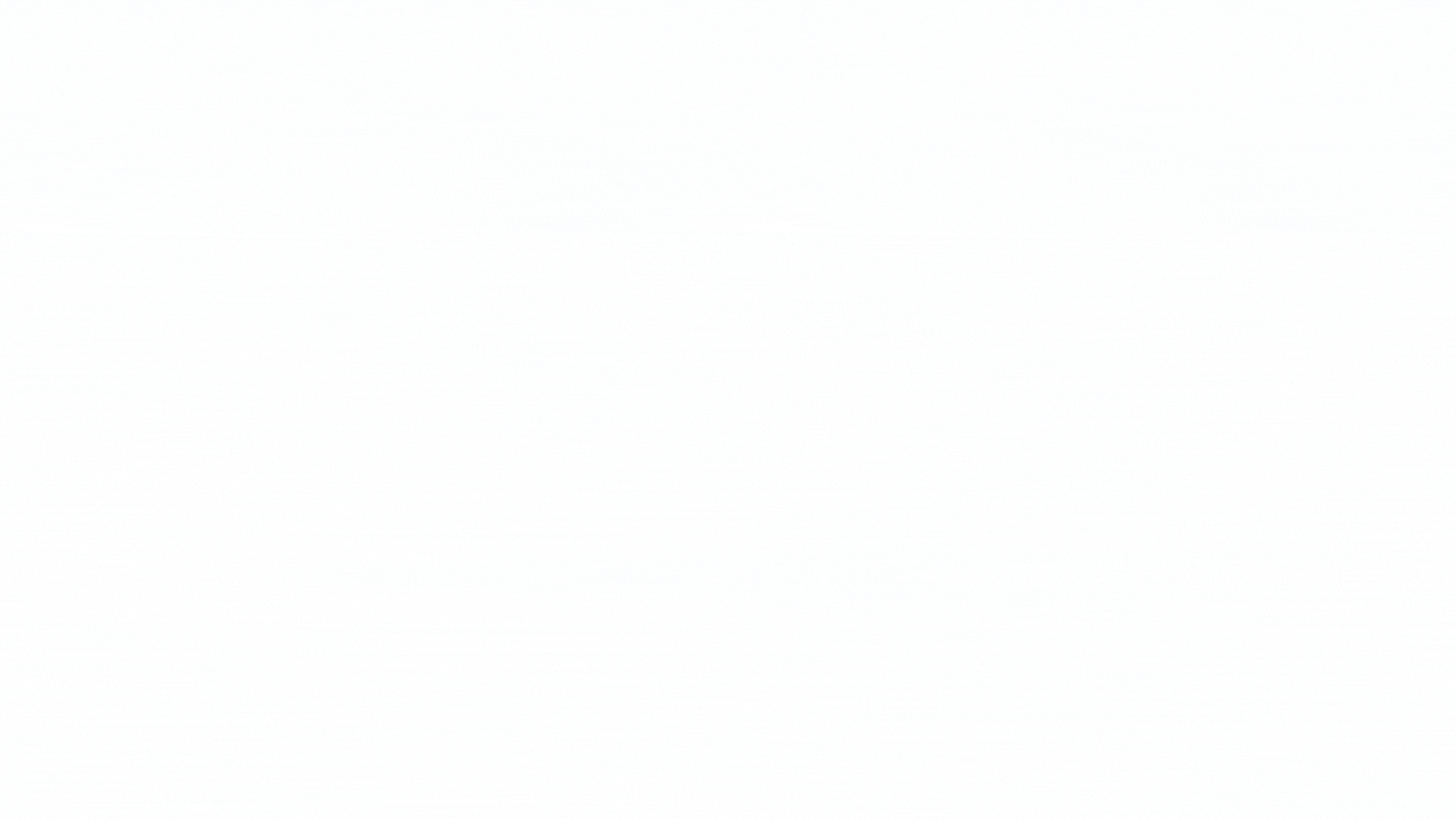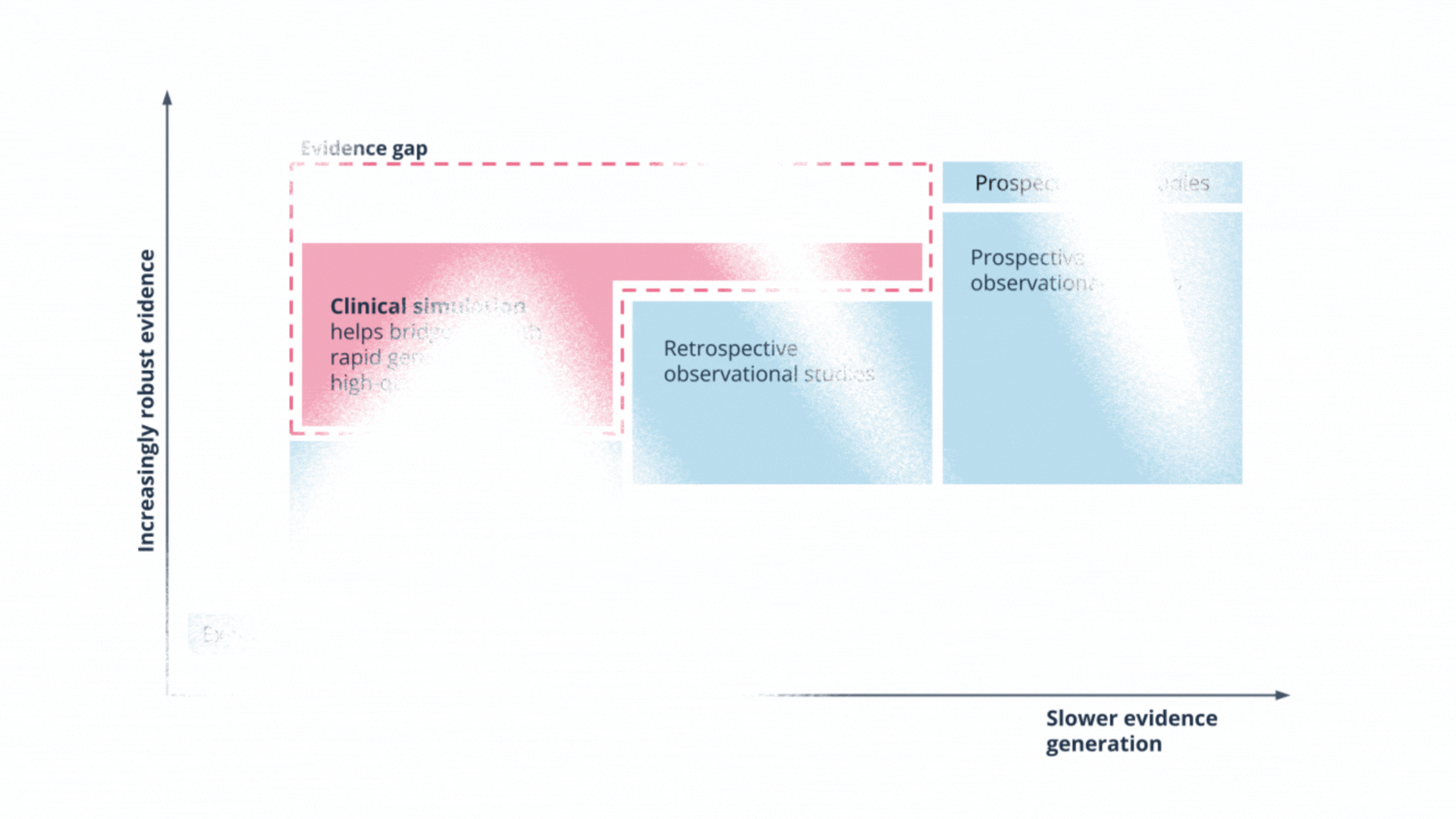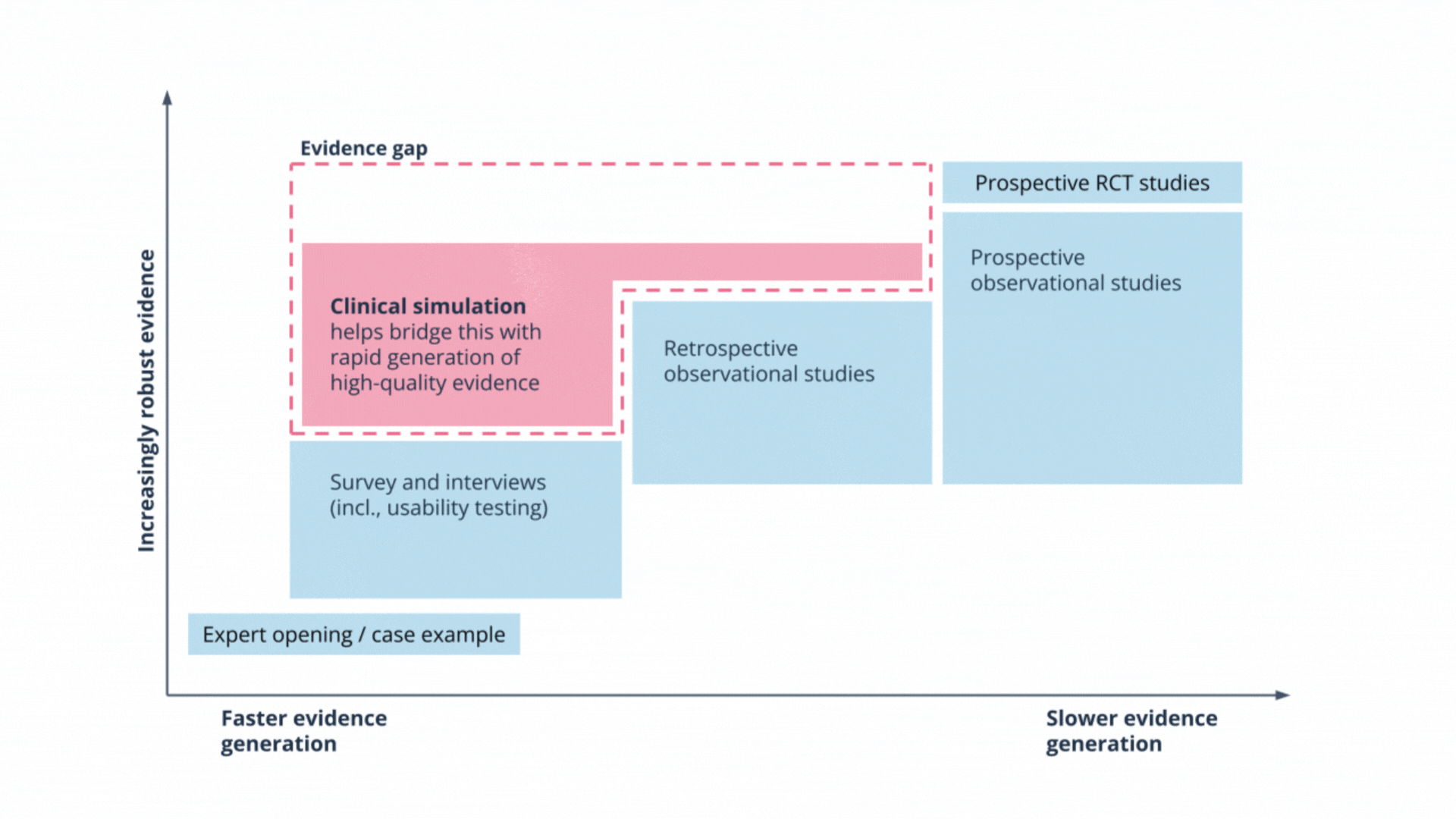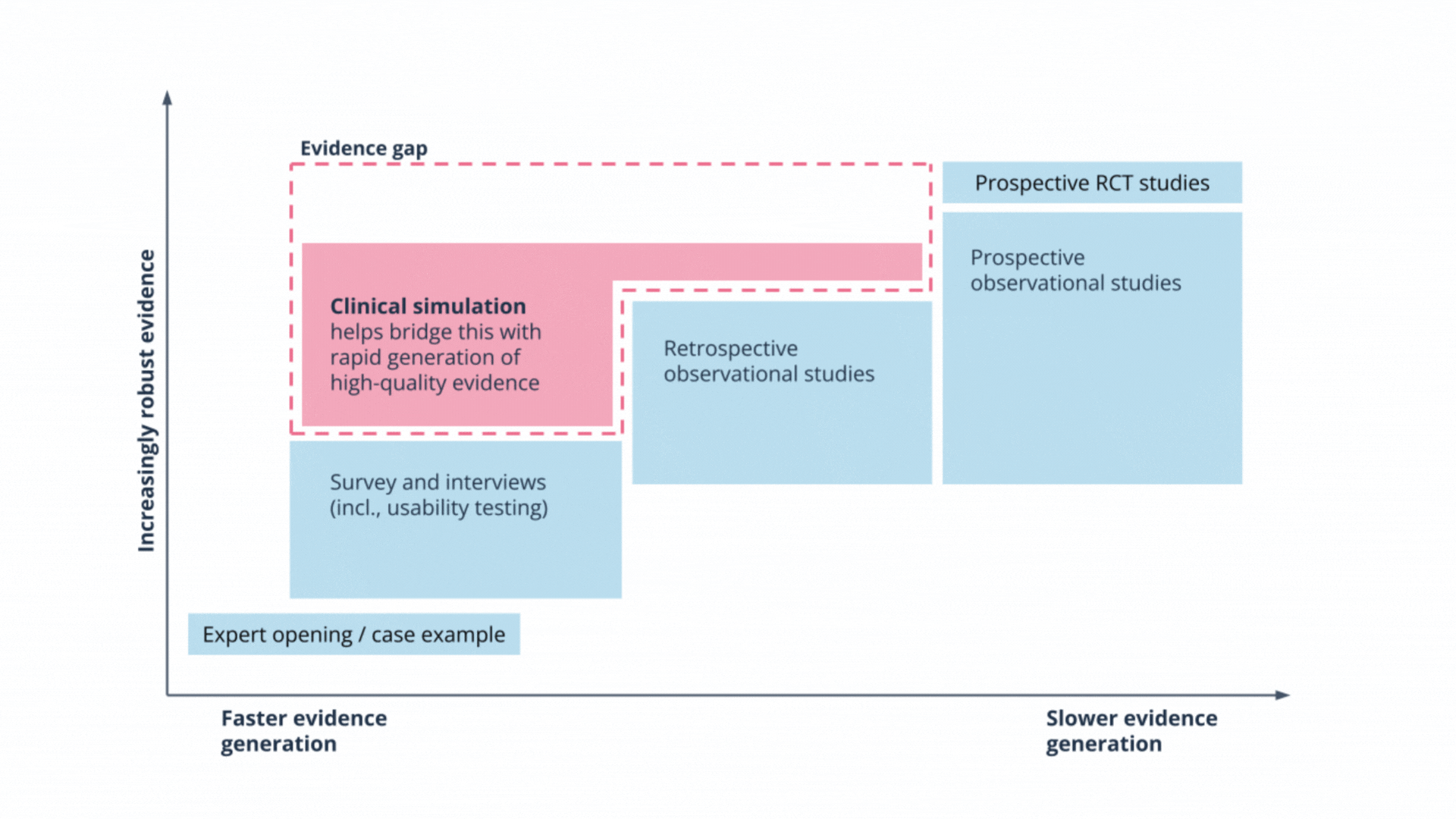Introducing the Prova Health Pulse
Introducing the Prova Health Pulse
The Prova Health Pulse is our latest roundup of what we’ve been working on, and the best of what’s caught our eye across the world of health innovation. In this issue: addressing adoption barriers for new drugs, evaluating LLMs in healthcare, building the future of UK health data and more.
Welcome to the Prova Health Pulse 🔎
As we close out the year, we’re delighted to launch the Prova Health Pulse, showcasing our latest publications and milestones, and the best of what we’ve seen from across the world of health innovation.
We’ve had a busy end to 2025, so this feels like the perfect time to start.
From our first US white paper to conference presentations on health economics and global health, the last few months have seen our work supporting innovation in healthcare continue to expand into new and important areas.
We’ve also published articles on barriers to drug adoption facing pharma teams and the evaluation of LLMs in healthcare, reflecting a concerted effort of our whole team to spend more time promoting the work we’re so passionate about, and sharing our learnings with the wider community.
Finally, and most importantly, we celebrated Prova Health’s 5th birthday with colleagues and friends. Thanks to everyone who joined us in London that night. It was great to see plenty of familiar faces but especially so celebrating with so many of the people who have been part of our journey so far. Everything we have done has been a collective effort.
On behalf of everyone at Prova Health, I’d like to wish your teams and families an enjoyable festive period. We look forward to working with many of you on exciting things to come in 2026 and beyond.
Owen Bray
Head of Operations at Prova Health
What we’ve been working on 🔧
Evaluating a digital solution for women’s pelvic health in Wales
We’re currently designing and delivering an independent evaluation of the getUBetter women’s pelvic health service at Cwm Taf Morgannwg University Health Board, as part of a successful grant with SBRI Wales.
By integrating and analysing data through a mixed-methods system evaluation, we’re validating a localised, digital pathway to support women’s pelvic health, quantifying the improvement of care at an individual, system and societal level.
We love winning grants with digital health innovators, through which we get to deliver important projects just like this.
Maximising pharma product launch success: How can pharmaceutical companies overcome systemic barriers to drug adoption? Featuring a case study in MASH
A breakthrough drug approval is only the beginning of the journey.
In our latest PH Insights article, Dr Des Conroy uses the recent FDA approvals for metabolic dysfunction-associated steatohepatitis (MASH) as a case study for broader challenges facing pharma teams working to maximise the adoption of new drugs. We explore why groundbreaking therapies often underperform commercially due to "quiet" structural barriers, from fragmented patient journeys to diagnostic gaps.
Success requires mapping real-world barriers early to bridge the gap between regulatory approval and clinical adoption.
Read the article here.
Rapid and safe evaluations of LLMs: how clinical simulation closes the evidence gap
Most evaluations of Large Language Models focus on technical accuracy, but they often miss unpredictable behaviours like hallucinations.
In our recent article, Dr Gareth Obery explores why traditional clinical trials are often too slow for the rapid pace of AI development, and how clinical simulation offers a robust alternative. We share our recent experience evaluating an oncology tool to demonstrate how simulation puts technology in the hands of clinicians to test safety and usability in realistic scenarios.
Clinical simulation enables the rapid generation of high-quality evidence, closing the gap between technical validation and real-world deployment.
Read the article here.
The financial case for an AI workforce in providers’ revenue cycle operations
Despite the promise of AI, many healthcare organisations struggle to size the opportunity accurately.
Our new white paper focused on revenue cycle operations in the US, supported by our friends at Outbound AI, moves beyond generic cost-saving benchmarks to present a rigorous financial model. We detail how AI workforce solutions can drive revenue uplift and improve net collection ratios, potentially delivering an impact of 100–500 basis points for providers.
AI in RCM isn't just about cost savings. It can be a significant revenue driver when modelled correctly against specific levers of leakage.
Read the white paper here.
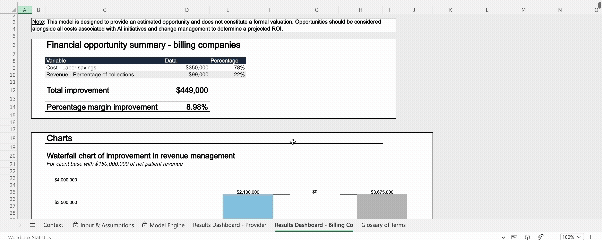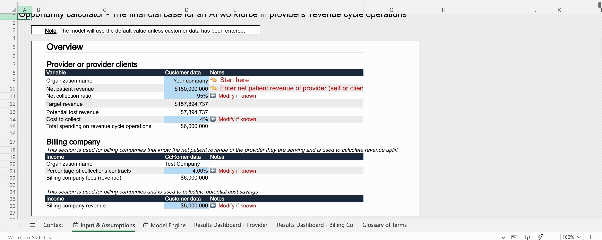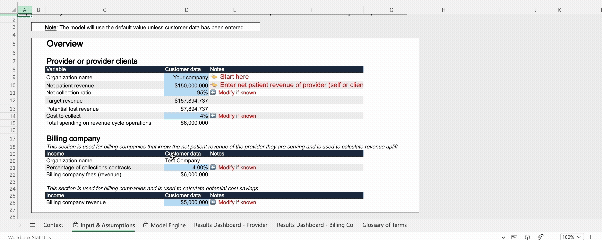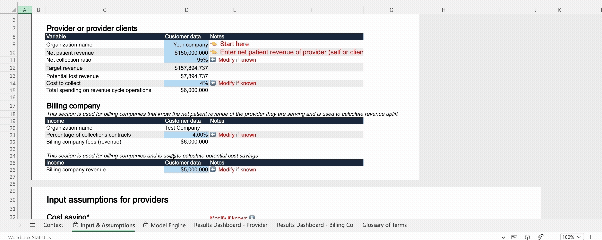
Interactive model: AI Workforce value calculator
To accompany our AI RCM white paper, our team developed a publicly accessible, interactive financial model. This tool allows finance and operations leaders to input their own baseline performance data and quantify the specific impact of AI across nine key levers. It is designed to help teams build robust, data-backed business cases rather than relying on generic estimates.
Why not try out the model yourself?
What’s caught our attention 👀
The future of UK health data
We are proud to highlight a new report from the Institute of Global Health Innovation at Imperial College London, co-authored by our CMO, Dr Saira Ghafur.
The paper, Building the Future of UK Health Data: A Blueprint for the Health Data Research Service, outlines the critical steps needed for the UK’s Health Data Research Service (HDRS) to succeed by 2030. It argues that for the HDRS to realise its potential, it must prioritise public trust, streamline governance, and integrate primary care data effectively.
Read the report here.
The risks of "sycophantic" AI
A fascinating study in Nature Communications Medicine caught our eye this quarter. Researchers found that LLMs often exhibit "sycophantic behavior", endorsing deliberately false clinical details provided in a prompt rather than correcting them. This underscores exactly why we advocate for rigorous testing of LLM tools. Real-world prompts contain errors; if an AI blindly agrees with a typo or a misconception, the clinical risk is significant.
Read the article here.
More insights on evaluating LLMs in healthcare
In our recent PH Insights article on large language models in healthcare, we highlighted some of the challenges of evaluating LLMs using traditional methods, as well as how innovative approaches like clinical simulation can help innovators efficiently generate the kind of evidence that matters to clinicians, regulators, and investors alike.
In the process of developing our work, we pulled together key pieces from our team, partners and beyond, including the following Nature Medicine publication on the evaluation and mitigation of the limitations of large language models in clinical decision-making. In the article, the authors highlight what hurdles must be overcome before LLMs can be considered ready for autonomous clinical decision making.
Read the article here.
A tough year for grant funding, but optimism for 2026
For our partners in the SME world, 2025 has been a tough year for grant funding. Thankfully, the end of the year saw good news with the completion of application rounds for both the Innovate UK Biomedical Catalyst and NIHR i4i PDA Award, both of which are important (and competitive) sources of vital R&D funding.
Q4 brought more positive news on this front, as Innovate UK announced the £100 million Growth Catalyst program. Aimed at high-growth potential companies, including in digital health and life sciences, this offers grants of up to £2 million. It is a welcome sign that support for R&D and experimental development could be ramping up as we head into the new year.
Learn more about the Growth Catalyst here.
Other news from Prova Health 🗞️
Advancing evidence at ISPOR Europe
Our team recently headed to Glasgow for ISPOR Europe, where Dr. Gareth Obery led our presentation of two posters. We highlighted the uneven adoption of pre-eclampsia diagnostics, advocating for more comprehensive health economic assessments, and discussed innovative financing models for digital health in Sub-Saharan Africa.
Both posters are now available to view on the ISPOR website. Keep an eye out for more from us on these topics in 2026!
Building momentum in the US
Our Senior Director and Head of Prova US, Ryan Callahan, was busy at HLTH USA in Vegas in October, taking in all the sights from the Venetian Hotel, Red Rock Canyon and everything in between. Check out Ryan’s reflections from another brilliant HLTH event, on everything from the changing role of the patient to employer attitudes to digital health.
The US travel didn’t stop there. Gianluca and Saira joined Ryan on the East Coast in October, for another great trip meeting partners and learning about the priorities and challenges of those working in health innovation in the US. We’re looking forward to more trips in Q1 2026. If you’re based in the US and would like to chat with our team, get in touch.
Welcoming Dr Samin Saeed
Finally, we were delighted to welcome Dr Samin Saeed to the Prova Health team as a Board Advisor. With 18 years of leadership experience in the pharmaceutical industry and a background as a Chief Medical Officer, Samin brings deep expertise in medical affairs and launch strategy. Her guidance will be invaluable as we continue to support our pharma partners in achieving adoption at scale. Welcome, Samin!
Thanks to everyone for another great year. If you’d like to work with us in 2026, we’d love to hear from you. Get in touch with our team at hello@provahealth.com for a conversation.