Maximising pharma product launch success: How can pharmaceutical companies overcome systemic barriers to drug adoption? Featuring a case study in MASH
Maximising pharma product launch success: How can pharmaceutical companies overcome systemic barriers to drug adoption? Featuring a case study in MASH
Even the most groundbreaking drugs rarely reach their full commercial potential because of adoption friction: the structural and logistical realities that slow or limit their integration into health systems. Achieving a successful pharma product launch is difficult; 1 in 2 new drugs fail to meet launch forecasts. The emergence of the first treatments for MASH serves as a key case study, illustrating how diagnostic gaps, workforce shortages, and fragmented care create friction that leads to delayed or limited uptake. To maximise commercial success and ensure new products perform on launch expectations, companies must identify, characterise, and overcome the specific systemic barriers in key markets.
When breakthrough treatments meet health system realities
Every year, new therapies promise to transform outcomes for patients, but history tells a consistent story: even the most groundbreaking drugs rarely reach their full clinical or commercial potential. Achieving a successful pharma product launch is difficult. 1 in 2 new drugs fail to meet launch forecasts (1).
The reason for this is adoption friction: the structural and logistical realities that slow or limit the integration of new therapies into health systems.
Examples include fragmented care pathways, workforce shortages, diagnostic gaps, and evolving guidelines that can make even well-evidenced treatments difficult to adopt at scale.
The impact is both clinical and commercial. Patients miss out on effective treatments, and pharmaceutical companies see delayed or limited uptake.
This excitement is perfectly captured by the evolving therapeutic area of metabolic dysfunction-associated steatohepatitis (MASH). The first approved therapies have finally emerged for MASH, representing a long-awaited breakthrough for millions of patients in a disease area previously defined by a lack of pharmacological options.
This makes MASH a prime case study for examining adoption friction. Despite scientific breakthroughs, the complex patient journey from diagnosis to treatment means these new drugs risk underperforming commercially and failing to reach patients at scale.
This underscores why identifying and addressing barriers to adoption early is a strategic imperative for the pharmaceutical industry and healthcare as a whole.
MASLD, MASH, fibrosis and cirrhosis
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common chronic liver disease, with a global prevalence of over 30% in the general population (up from 25% in 2016) (2,3).
Previously known as non-alcoholic fatty liver disease (NAFLD), MASLD is defined by excess fat in the liver occurring with at least one cardiometabolic risk factor and without harmful alcohol intake.
MASLD encompasses a spectrum of disease, progressing from the accumulation of fat (steatosis) to metabolic dysfunction-associated steatohepatitis (MASH), the critical inflammatory stage at which the disease becomes damaging, driving worsening scarring (fibrosis) of the liver, which may eventually be irreversible (cirrhosis).
A shift in liver disease treatment
For decades, clinical management of MASLD was defined by a complete lack of pharmacological options. Treatment was confined to lifestyle modifications, even as the disease progressed through to MASH, liver fibrosis and ultimately, the irreversible damage of cirrhosis.
That era is now decisively over. The recent FDA approval of therapies like resmetirom (Madrigal) and semaglutide (Novo Nordisk) marks a critical turning point, taking MASH from an area with no effective drugs to one with real promise for patients.
The emergence of these drugs represents a major victory, but it is only the first step. The real challenge now lies in ensuring these vital treatments reach every patient who needs them.
The first new pharma products for MASH
Resmetirom and semaglutide are now approved by the FDA for the treatment of MASH with moderate-to-severe fibrosis. This represents a breakthrough for a previously underserved patient population.
Resmetirom
Resmetirom is a liver-directed thyroid hormone receptor-beta (THR-β) agonist. It mimics thyroid hormone, binding to THR-β receptors in the liver, leading to a reduction in steatosis and fibrosis. This drug was approved in the US in March 2024, and conditional marketing authorisation was granted in the EU in August 2025.
Semaglutide
Semaglutide is a GLP-1 agonist, well known for its weight-loss effects. It mimics the natural incretin hormone GLP-1 to regulate blood sugar and increase satiety. The drug has beneficial effects both on the liver damage that occurs in MASH and on associated cardiometabolic conditions. Data from an ongoing Phase 3 trial (ESSENCE) shows that treatment led to the resolution of steatohepatitis, regression of fibrosis, and an improvement in cardiometabolic parameters.
Looking ahead: Future pharmaceutical product launches
While resmetirom and semaglutide are first to market, the MASH treatment landscape is expanding. Many drugs are currently in clinical trials, including next-generation incretin mimetics, peroxisome proliferator-activated receptor (PPAR) agonists, fibroblast growth factor 21 (FGF21) analogues, and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
Companies with potential MASH treatments in the pipeline include Merck, Pfizer, Astrazeneca, Eli Lilly, GSK, Boehringer Ingelheim, Hanmi, Inventiva, Altimmune, and 89bio. Therefore, the range of pharmacological options will likely continue to grow.
What are the potential barriers to adoption in MASH?
While the emergence of new treatments is promising, there are challenges ahead for companies bringing drugs to market for MASH. It is well established that a significant proportion of drugs underperform on launch expectations.
This occurs for multiple reasons, and many relate to specific, real-world barriers that prevent a drug from being adopted into standard clinical practice.
New MASH treatments are not emerging into a simple, linear patient journey, but a complex picture with several potential barriers to timely and effective adoption.
Below are some examples of potential sources of friction.
A primary care diagnosis gap
MASH is a “silent” condition, highly prevalent in high-risk populations managed by primary care providers (PCPs).
In liver biopsy-based studies, the prevalence of MASLD in patients with type 2 diabetes (T2D) has been shown to be over 90% (4). However, PCPs may be less confident and underequipped when it comes to identifying MASH and liver fibrosis.
This may create a pre-referral bottleneck, where many eligible patients are never diagnosed with the stage F2-F3 fibrosis for which treatment is indicated.
No clear standard for non-invasive testing
Liver biopsy remains the gold standard for diagnosing fibrosis, but it is an invasive and impractical procedure for routine clinical use.
While guidelines support the use of non-invasive tests to identify patients for treatment, more evidence is needed to determine which non-invasive modalities are best.
The lack of a clear, best-in-class non-invasive diagnostic may present a barrier to timely identification of patients who can benefit from treatment.
A fragmented patient journey
MASH has strong multi-directional links with conditions like T2D and obesity, so patients are often managed by multiple specialists.
A lack of clear, integrated pathways facilitating communication between specialists leads to diagnostic delays and fragmentation of care, which leads to missed opportunities for treatment.
Research indicates that approximately 20 million people in the US, UK, Germany and France have MASH, but only 2.5 million people have been diagnosed. This means the vast majority of cases have not been identified (5).
A specialist care bottleneck
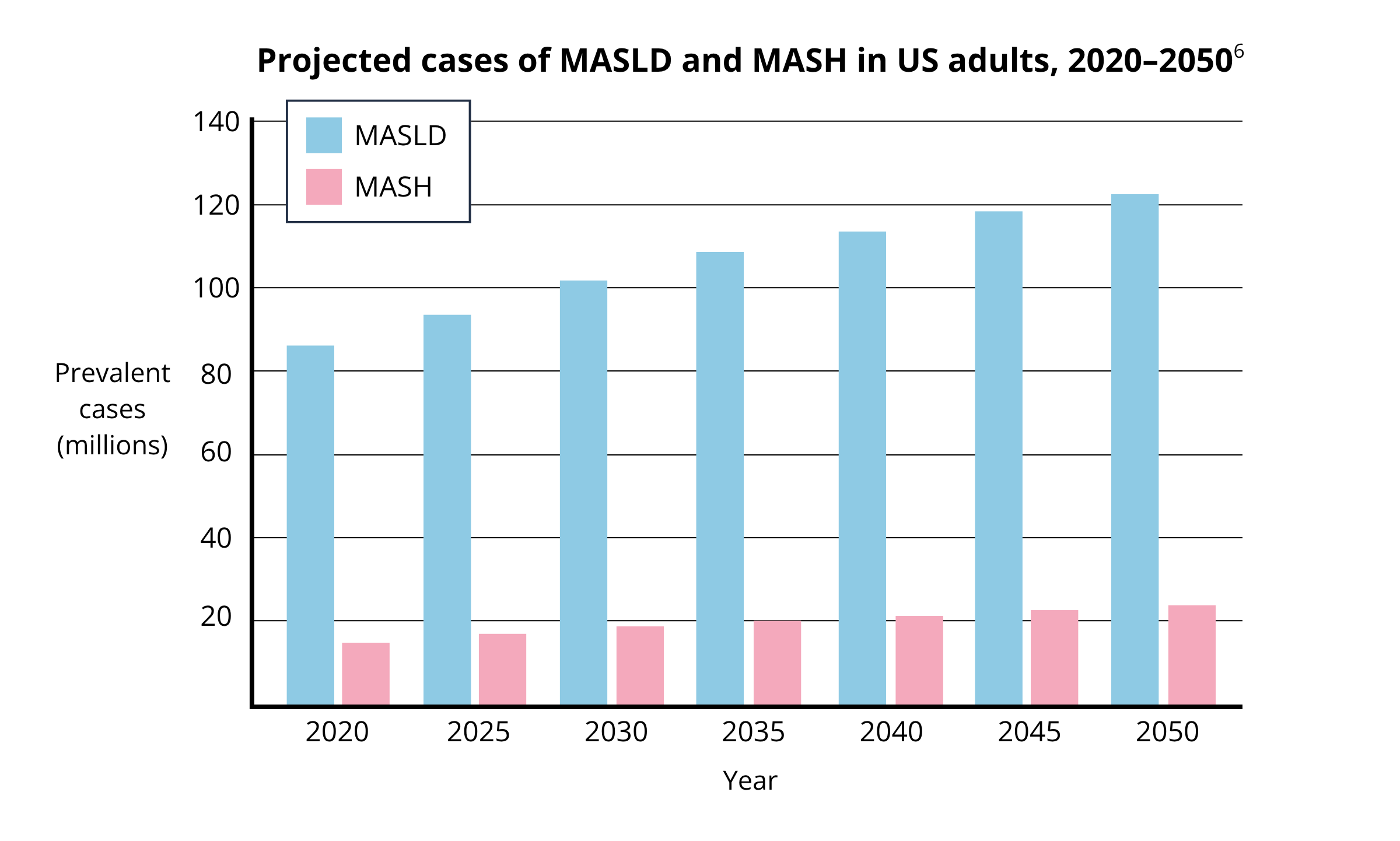
Deficits in the specialist clinical workforce can be a rate-limiting step for drug adoption. Take the United States for example, where the adult population with MASH and significant fibrosis (indicating pharmacological treatment) is projected to increase to 11.7 million by 2050 (6). At the same time, models predict a 35% shortage of hepatologists by 2033 (7).
This is exacerbated in the US by some payer criteria that mandate specialist oversight for therapy, meaning consultation with a clinical specialist can become a constraint on the number of patients receiving optimal treatment. This directly impacts market access.
Evolving guidelines
Clinical guidelines are in a state of flux, mirroring evolving evidence and drug approvals. While clinical bodies are updating their guidelines in line with new developments, the pace of change may itself be a barrier to adoption: clinicians could be hesitant to adopt new therapies while awaiting established guidelines, and may find themselves navigating ambiguous or conflicting recommendations.
Effective launch strategy: How can we overcome systemic barriers to maximise the success and impact of new drugs?
The approval of new MASH treatments is a major victory, but the clinical reality shows that this is only the first step. The true challenge for companies is eliminating the structural and logistical friction that prevents eligible patients from accessing therapy.
To successfully transition from a breakthrough approval to widespread clinical adoption, companies must focus on the following strategic imperative: Identifying, characterising, and overcoming the specific systemic barriers in key markets.
This requires going beyond general awareness campaigns and getting precise answers to questions like:
Where does the patient journey break down due to a specialist shortage?
How many patients are lost due to a lack of non-invasive tests in some settings?
What interventions are needed to bridge the knowledge gap for non-specialist clinicians (e.g., PCPs)?
How can pharmaceutical companies plan and execute high impact strategies for launch success, informed by deep health system expertise?
At Prova Health, our expertise lies in helping you answer these questions. We support some of the world's leading pharma companies to understand the clinical reality on the ground by mapping clinical pathways and identifying barriers to adoption.
Leveraging these insights, we develop clear roadmaps with our clients to overcome these barriers, maximise the impact of product launches, and get new treatments to more patients who need them.
If your team is working to bridge the gap between breakthrough approval and real-world adoption, we’d love to discuss how we can support your next launch. Get in touch with our team at hello@provahealth.com.
References
Whitman M, Stevens J, Burgess T, Stubbs C, Sahastrabudhe A. 2025 ZS biopharma commercialization report: Trends that are modernizing the go-to-market model [Internet]. Evanston (IL): ZS; 2025 [cited 2025 Nov 11]. Available from: https://www.zs.com/content/dam/pdfs/2025-ZS-biopharma-commercialization-report.pdf
Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023 Apr 1;77(4):1335-47.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
Leith D, Lin YY, Brennan P. Metabolic dysfunction-associated steatotic liver disease and type 2 diabetes: a deadly synergy. TouchREVIEWS in endocrinology. 2024 Apr 23;20(2):5.
Lazarus JV, Brennan PN, Mark HE, Alazawi W, Allen AM, Byrne CD, Castera L, Caussy C, Cusi K, Grajower MM, Kopka CJ. A call for doubling the diagnostic rate of at-risk metabolic dysfunction-associated steatohepatitis. The Lancet Regional Health–Europe. 2025 Jul 1;54.
Le P, Tatar M, Dasarathy S, Alkhouri N, Herman WH, Taksler GB, Deshpande A, Ye W, Adekunle OA, McCullough A, Rothberg MB. Estimated Burden of Metabolic Dysfunction–Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Network Open. 2025 Jan 2;8(1):e2454707-.
Russo MW, Fix OK, Koteish AA, Duggan K, Ditmyer M, Fuchs M, Chung RT, Reddy G. Modeling the hepatology workforce in the United States: a predicted critical shortage. Hepatology. 2020 Oct;72(4):1444-54.
About the author - Dr Des Conroy
Des is a medical doctor and Senior Consultant at Prova Health, where he leads the company's capability building practice and a diverse portfolio of projects for pharma and medtech clients. Drawing on his experience from clinical practice, healthtech, and life sciences consulting, he supports clients to deeply understand the diseases their solutions address, and the clinical and operational realities shaping how patient care is delivered in diverse healthcare systems. Des has a special interest in translating these insights into high-impact capability building programmes that equip teams for success.